The periodic table visually shows the elements that make up everything we see around us. It is a key concept in chemistry which needs to be taught to our students. I prefer to introduce the idea of the periodic table in the elementary years. Then, teach the relationships and categories of the periodic table during the middle school years. That way, the students go into high school chemistry already familiar with the periodic table, so that they can really focus on learning the chemistry and mathematics behind the periodic table.
What is the periodic table?
The periodic table is a systematic arrangement of the elements in order of increasing atomic number. It is designed to group elements with similar properties together.
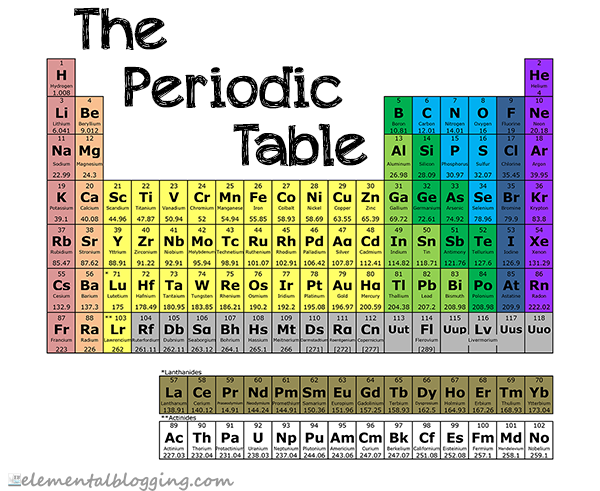
The periodic table gives the following information for each element…
- The atomic number, which is the number of protons that can be found in the nucleus of an atom.
- The atomic mass, which is the total weight of the protons, neutrons and electrons in a given atom. Sometimes this can vary if there are isotopes of the element, so the atomic mass given on the periodic table is an average of those varying weights.
- The symbol, which is the 1, 2 or 3-letter code that scientists use for the element. This code is accepted internationally to remove language barriers when discussing chemical compounds. Some are easy, like O for Oxygen; some make less sense, like Pb for Lead. This is because the symbol is typically based on the Latin name for the element, which in the case of lead is plumbum. Chemists use the symbol of an element when referring to it in a compound or equation, so these are important to know.
As you move from left to right on the table, the atomic number and atomic mass of the element increases. The same is true as you travel down the periodic table.
Who is responsible for the periodic table?
The original periodic table was created by Russian chemist Dmitri Mendeleev. He wrote it almost 30 years before Thomson discovered the electron, close to 45 years before Rutherford found the nucleus of an atom and over 50 years before scientists determined that the proton and neutron made up the nucleus of the atom. He proposed his primitive version of today’s periodic table as a result of writing a textbook on general chemistry. Through his research he was struck by the fact that the elements chemical properties varied with the atomic mass, so he drew up a table to show these relationships.

Mendeleev’s 1871 Periodic Table, photo credit
In a stroke of genius, he left gaps for elements that had not yet been discovered and even went so far as to predict the properties of those missing elements. When they were finally discovered, their properties were very similar to what Mendeleev had predicted. Even though our modern day table looks quite a bit different from what Mendeleev drew, we still give him credit for the original idea of the periodic table.
How to teach the periodic table?
You can introduce the periodic table informally through books and then use games to help your students learn the included elements, or you can chose to study chemistry more formally using a pre-planned program. Here are two free pdf’s of the periodic table to get you started:
The following books are two of my favorites to learn more about the periodic table:
- The Mystery of the Periodic Table (narrative-style story of the elements)
- Fizz, Bubble, Flash (great resource for studying the elements of the periodic table, includes experiments)
To help your students learn more about the elements of the periodic table, you can play one of the two games below:
- Guess the Element: Make your own set of element cards or purchase a set. Set the deck of element cards face down in the center of the players. Have the first player choose a card and begin to read the information on the card, i.e. “This element is 8th on the periodic table, this element is essential to life, it is in the air, it weighs 16.02 g, it discovered by Joseph Priestly” and so on. The other players try to call out what they element is as soon as they figure it out. The first person to guess which element it is wins a point. If no one guesses the element, then no one gets the point. The first person to reach 10 points is the winner of the game.
- Periodic Table Match-up: You will need one set of element cards (homemade or purchased), a copy of the periodic table and pen for each student. Choose one player to be the caller. Have them choose an element from the deck of cards and call out its name. Then the caller will begin counting to 12, if the players find the element within 6 seconds, they put a check on the element. If they find it within 12 seconds, they will circle the element. Once the caller reaches 12, they will give the atomic number of the element and the players who haven’t found the element yet, will put an x over it. Checks are worth 2 points, circles are worth 1 point and x’s are worth no points. The game ends once all of the elements have been called. The player with the highest points wins the game.
Finally, if you choose to use a program to learn more about the periodic table, Elemental Science offers the following programs to help you out:
- Lapbooking through the Periodic Table
- Chemistry for the Grammar Stage
- Chemistry for the Logic Stage (Coming in early August 2013)
No matter how you choose to share the periodic table with your students, it will serve to increase their understanding of chemistry deepen their appreciation of the elements that make up world around them.
Do you want more? You can sign up to receive new posts via email!







Join the Community!