What is an element? What is an atom? How do an element and atom differ? Today I’m getting a bit technical as I share about these two essential concepts in chemistry.
The Element:
 Elements are a substances made up of one type of atom that cannot be broken down by chemical reaction to form a simpler substance. In other words, they are a type of matter which cannot be broken down into two or more substances. There are 118 known elements at the time of this article and 92 of them can be found naturally. The remainder must be synthetically produced, usually by man-made nuclear reactions.
Elements are a substances made up of one type of atom that cannot be broken down by chemical reaction to form a simpler substance. In other words, they are a type of matter which cannot be broken down into two or more substances. There are 118 known elements at the time of this article and 92 of them can be found naturally. The remainder must be synthetically produced, usually by man-made nuclear reactions.
Some elements are very familiar to us, such as gold, copper and iron. Charcoal is composed or nearly pure carbon, aluminum is used for foil and silicone is used in nearly every electronic on the market. Other elements are necessary to life, such as oxygen, nitrogen and phosphorus. Each of the elements can be found arranged according to atomic number on the periodic table.
Gold was one of the first elements to be discovered and used. It has been used for much of recorded history and was used extensively by the ancient Egyptians in the tombs of their pharaohs. The Bible records that Tubal-Cain used the element iron. However, the first concept of an element was described by the ancient Greek philosophers, who said that there were four elements, fire, earth, water and air. They believed that all substances were a combination of these four elements.
In 1661, Robert Boyle showed that there were more than just the four classical elements, but it was not until 1789 that the first list of elements, which contained only 33 elements, was written by Antoine Lavoisier. In 1869, Dmitri Mendeleev was the first to organize the 66 known elements of his time into a table that displayed the relationships between those elements. This table became the basis for our modern periodic table. As the field of science moved into the modern era, new instruments such as the spectroscope have allowed for more and more elements to be discovered.
The Atom:
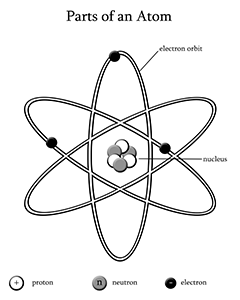 The atom is the smallest particle of an element that still retain the chemical properties of that element. The Greeks were the first to discuss the concept of an atom. They believed that matter could be cut into smaller and smaller pieces, but that eventually you would get to a piece that could not be cut. So, the word atom comes from the Greek word, atomos, which means “uncuttable”. It was not until 1808 that John Dalton, an English scientist and schoolteacher, developed a theory about the how atoms behave. His theory said that an element is composed of tiny particles called atoms, in an ordinary chemical reaction, no atom of an element disappears and compounds are formed when atoms of two or more elements combine. So on the basis of his theory; scientists were now able to say that the atom was the smallest particle of an element.
The atom is the smallest particle of an element that still retain the chemical properties of that element. The Greeks were the first to discuss the concept of an atom. They believed that matter could be cut into smaller and smaller pieces, but that eventually you would get to a piece that could not be cut. So, the word atom comes from the Greek word, atomos, which means “uncuttable”. It was not until 1808 that John Dalton, an English scientist and schoolteacher, developed a theory about the how atoms behave. His theory said that an element is composed of tiny particles called atoms, in an ordinary chemical reaction, no atom of an element disappears and compounds are formed when atoms of two or more elements combine. So on the basis of his theory; scientists were now able to say that the atom was the smallest particle of an element.
Modern atomic theory is very similar to what Dalton proposed, except that now we know of the sub-particles that compose an atom along with how the atom is structured. The atom is composed of three smaller subatomic particles, called the proton, neutron and electron. The proton is a positively charged particle that resides in the nucleus at the center of an atom. The neutron is a particle with no charge that also resides in the nucleus of an atom. The electron is a negatively charged particle that resides in a cloud, or electron shell, around the nucleus.
Atoms have an equal number of protons and electrons, which gives them no net charge. In other words, the positive charges from the protons are cancelled out by the negative charges of the electrons within the atom. Generally an atom of a given element has the same number of neutrons as protons, but there are exceptions. Some atoms have additional neutrons in their nucleus and we call these atoms isotopes of the element. These isotopes have the same atomic number, but different atomic mass. Remember that the atomic number is the number of protons in an element and atomic mass is the total weight of the protons, neutrons and electrons in an element. So it makes sense that an isotope would have the same atomic number, but a different atomic mass from the original element.
Atom or Element?
So how do atoms and elements differ? Elements are substances that are made up of one type of atom, while atoms are the smallest particles of an element that retains the chemical properties of the element. In other words an element is composed of one or more of the same type of atom. So, when you hold a lump of iron ore, you are holding the element iron which contains billions of iron atoms.
Do you want more? You can sign up to receive new posts via email.






Join the Community!